Abstract
Introduction: In acute leukemia with KMT2A (MLL1)-rearrangement (KMT2Ar) or NPM1 mutation (mNPM1 or NPM1c), menin inhibition by SNDX-5613 (revumenib) disrupts both wild-type KMT2A and KMT2A fusion protein complexes that drive leukemogenesis. Results of the Ph 1 dose escalation portion of the AUGMENT-101 (NCT04065399) trial demonstrated high response rates and manageable safety profile, supporting the initiation of the currently enrolling Phase 2 cohorts at the RP2D of 163 mg q12 hr with strong CYP3A4 inhibitors. Here, we provide an update on r/r KMT2Ar or mNPM1 patients in Arms A and B of the Ph 1 study who proceeded to hematopoietic stem cell transplant (HSCT) in remission (CR+CRh+CRp). We also describe the use of SNDX-5613 in 3 patients as maintenance therapy following HSCT or stem cell boost.
Methods: Arms A and B of the AUGMENT-101 study enrolled patients >= age 30 days with r/r AML in two parallel dose-escalation cohorts: patients not taking (Arm A) or taking (Arm B) strong CYP3A4 inhibitors, with SNDX-5613 dosed orally q12h in continuous 28-day cycles. Patients under 40 kg received BSA-based dosing, with a liquid formulation if needed. Per investigator discretion, patients could proceed to HSCT after discontinuation of SNDX-5613. Patients proceeding to transplant in remission (CR, CRh, CRp) were followed for safety for 30 days after the last dose of SNDX-5613, and for survival/disease assessment until progression. The results of this follow-up are reported here as of the data-cut date of Mar 31, 2022. 3 patients were treated with SNDX-5613 maintenance post-HSCT or stem cell boost on compassionate use single-patient protocols (SPPs). Data is more limited on these subjects with some data derived from investigator reports.
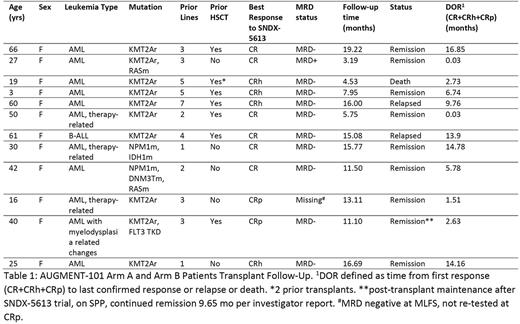
Results: 68 patients were treated on Arm A and B, 60 with r/r KMT2Ar or mNPM1 leukemia. Patients had a median of 4 lines of prior therapy, including 46% having undergone prior HSCT. The overall response rate (CR+CRh+CRp+CRi/MLFS [ORR]) in the efficacy population (KMT2Ar and mNPM1) was 53% (32/60); the rate of CR+CRh+CRp was 38% (23/60) with a CR/CRh rate of 30% (18/60 [95% CI: 18.8, 43.2]). The MRD negative rate among pts with CR/CRh/CRp was 78% (18/23). 12 patients proceeded to HSCT while in remission (CR, CRh, CRp) following SNDX-5613 monotherapy (Table 1). 10/12 (83%) of those patients were MRD negative per local flow cytometry. 7/12 (58%) had received at least 1 HSCT prior to entering the AUGMENT-101 study, with 1 patient having received 2 prior HSCT. 9/12 remained in remission as of the data cut with median follow-up 12.3 months (range 3.2-19.2), and 4 patients had remissions longer than 1 year. No new safety signals were identified. There was one death, due to sepsis, in a patient in remission post HSCT at approximately day 60, following a 3rd HSCT; they had not resumed SNDX-5613 post-transplant.
3 patients were treated with SNDX-5613 maintenance post-HSCT or stem cell boost on SPPs. 1 was among the 12 patients described in Table 1: a 40 yo F with KMT2Ar AML with FLT3 TKD, and 3 prior lines of therapy including 7+3+midostaurin and HSCT. She achieved CRp MRD- with SNDX-5613, proceeded to a second HSCT, followed by 2 cycles of azacytidine for 5 days, and then SNDX-5613 maintenance. She remained in remission at last follow-up almost 10 mo since her CRp. In addition, a 71 yo F with KMT2Ar AML achieved CRh MRD- on AUGMENT-101 and proceeded to a non-myeloablative stem cell boost, followed by SNDX-5613 maintenance, with a 14.5 mo remission. Finally, a 22yo F with KMT2Ar AML with 6 prior lines of therapy including 2 prior HSCTs became CRp MRD- on AUGMENT-101. She then had a molecular relapse and received CPX-351, gemtuzumab, venetoclax, and azacytidine, a 3rd allo-HSCT, and due to persistent positive MRD by flow, she received a donor lymphocyte infusion. She started SNDX-5613 maintenance on Day +111 post 3rd allo-HSCT. She was confirmed MRD negative by flow/PCR after 2 cycles SNDX-5613, and continues in remission more than 1 year.
Conclusion: In SNDX-5613 patients proceeding to transplant, durable remissions occurred across a range of heavily pre-treated patients. In addition to patients achieving CR/CRh, two patients with CRp also had ongoing remissions post-transplant. AUGMENT-101 continues to enroll patients, agnostic of transplant eligibility, with the option for SNDX-5613 post-transplant maintenance.
Disclosures
Issa:Novartis, Kura Oncology, Nuprobe: Consultancy; Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding. Stein:Astellas Pharmaceutical, Agios Pharmaceuticals, and Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen, AbbVie, Seattle Genetics, and Biotheryx: Consultancy; Syndax: Consultancy, Research Funding; PTC Therapeutics and Syros: Membership on an entity's Board of Directors or advisory committees; PinotBio, Bristol Myers Squibb, Jazz Pharmaceuticals, Foghorn Therapeutics, Blueprint Medicines, Gilead Sciences, Janssen Pharmaceuticals: Consultancy; Bayer: Research Funding; Daiichi-Sankyo, Celgene Pharmaceuticals, and Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Auron Therapeutics: Current equity holder in private company. Arellano:Kite, a Gilead Company: Consultancy, Research Funding; Syndax Pharmaceuticals: Consultancy. Žucenka:Astellas: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: Travel Expenses; Abbvie: Consultancy, Honoraria, Other: Travel Expenses; Pfizer: Consultancy. Khera:Incyte: Consultancy; Optum: Honoraria. Stone:Novartis: Consultancy; Janssen: Consultancy; Jazz: Consultancy; Boston Pharmaceuticals: Consultancy; Epizyme: Consultancy; Elevate Bio: Consultancy; BMS: Consultancy; GSK: Consultancy; Innate: Consultancy; Kura Oncology: Consultancy; BerGenBio: Consultancy; Apteva: Consultancy; Aprea: Consultancy; Arog: Consultancy, Research Funding; Astellas: Consultancy; Foghorn Therapeutics: Consultancy; Gemoab: Consultancy; Actinium: Consultancy; Abbvie: Consultancy, Research Funding; Syros: Consultancy; OncoNova: Consultancy; Syndax: Consultancy; Syntrix: Consultancy; Takeda: Consultancy. Thirman:AbbVie, AstraZeneca, Celgene,Janssen, Pharmacyclics, Roche/Genentech: Consultancy; AbbVie,Gilead Sciences,Janssen,Merck,Pharmacyclics, Syndax, TG Therapeutics, Tolero Pharmaceuticals.: Consultancy, Research Funding. DiPersio:Amphivena Therapeutics: Research Funding; NeoImmune Tech: Research Funding; Macrogenics: Research Funding; BioLineRx, Ltd.: Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; WUGEN: Current equity holder in private company, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Gu:Syndax: Current Employment. Bagley:Syndax: Current Employment. Rosen:Syndax: Current Employment. Tamang:Syndax: Current Employment. Dishman:Syndax: Current Employment. Scalera:Syndax: Current Employment. Meyers:Syndax: Current Employment. Madigan:Syndax: Current Employment. McNeer:Syndax: Current Employment. Aldoss:Autolus Limited: Consultancy; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Kite: Consultancy; AbbVie: Consultancy, Research Funding; Amgen: Consultancy; Agios: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal